
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Role of SUMO-Specific Protease 2 in Leptin-Induced Fatty Acid Metabolism in White Adipocytes
- Praise Chanmee Kim, Ji Seon Lee, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2023;47(3):382-393. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0156
- 3,191 View
- 158 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
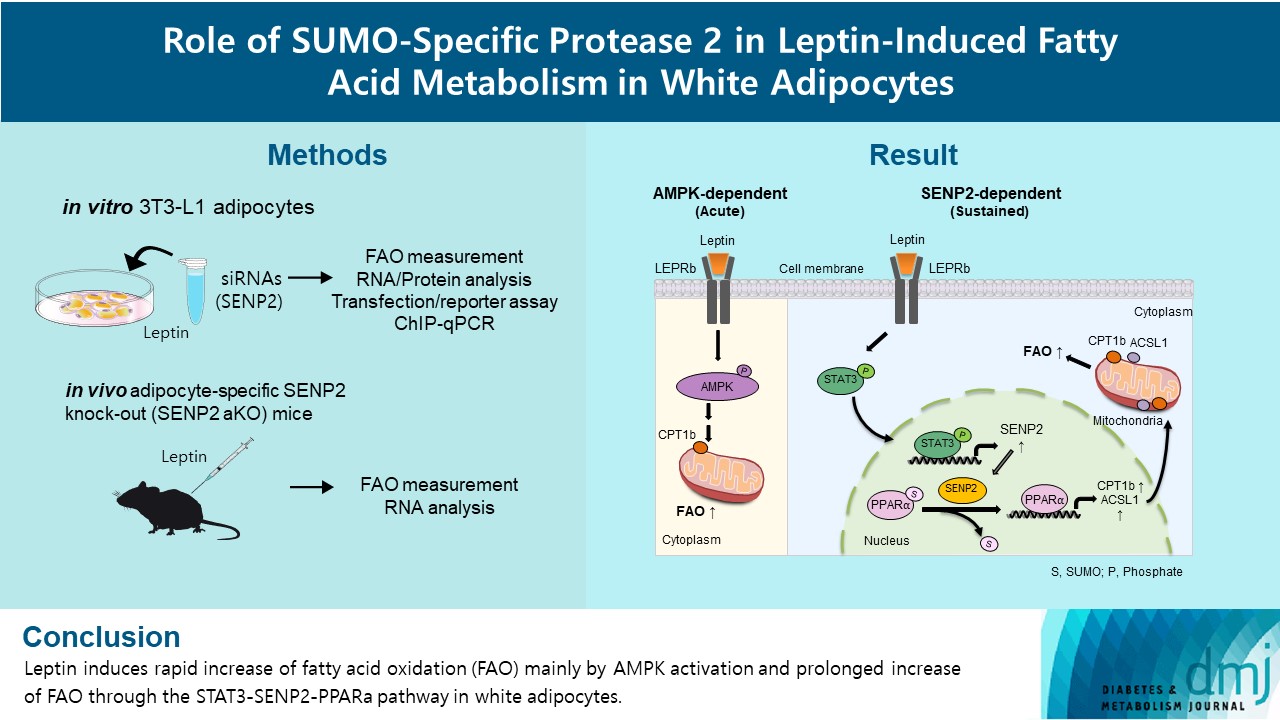
Leptin is a 16-kDa fat-derived hormone with a primary role in controlling adipose tissue levels. Leptin increases fatty acid oxidation (FAO) acutely through adenosine monophosphate-activated protein kinase (AMPK) and on delay through the SUMO-specific protease 2 (SENP2)–peroxisome proliferator-activated receptor δ/γ (PPARδ/γ) pathway in skeletal muscle. Leptin also directly increases FAO and decreases lipogenesis in adipocytes; however, the mechanism behind these effects remains unknown. Here, we investigated the role of SENP2 in the regulation of fatty acid metabolism by leptin in adipocytes and white adipose tissues.
Methods
The effects of leptin mediated by SENP2 on fatty acid metabolism were tested by siRNA-mediated knockdown in 3T3-L1 adipocytes. The role of SENP2 was confirmed in vivo using adipocyte-specific Senp2 knockout (Senp2-aKO) mice. We revealed the molecular mechanism involved in the leptin-induced transcriptional regulation of carnitine palmitoyl transferase 1b (Cpt1b) and long-chain acyl-coenzyme A synthetase 1 (Acsl1) using transfection/reporter assays and chromatin immunoprecipitation.
Results
SENP2 mediated the increased expression of FAO-associated enzymes, CPT1b and ACSL1, which peaked 24 hours after leptin treatment in adipocytes. In contrast, leptin stimulated FAO through AMPK during the initial several hours after treatment. In white adipose tissues, FAO and mRNA levels of Cpt1b and Acsl1 were increased by 2-fold 24 hours after leptin injection in control mice but not in Senp2-aKO mice. Leptin increased PPARα binding to the Cpt1b and Acsl1 promoters in adipocytes through SENP2.
Conclusion
These results suggest that the SENP2-PPARα pathway plays an important role in leptin-induced FAO in white adipocytes. -
Citations
Citations to this article as recorded by- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
Tingting Li, Haidong Wei, Shijie Zhang, Xiaotao Liu, Lu Xing, Yuanyuan Liu, Rixin Gong, Jianhong Li
Poultry Science.2024; 103(1): 103190. CrossRef
- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
- Pathophysiology
- Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Hong Il Kim, Ji Seon Lee, Byung Kook Kwak, Won Min Hwang, Min Joo Kim, Young-Bum Kim, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2019;43(6):854-866. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0179
- 6,649 View
- 115 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
Methods NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
Results We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
Conclusion This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
-
Citations
Citations to this article as recorded by- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
Si-min Zhou, Xin-ming Yao, Yi Cheng, Yu-jie Xing, Yue Sun, Qiang Hua, Shu-jun Wan, Xiang-jian Meng
Heliyon.2024; 10(2): e24432. CrossRef - Reduced Expression Level of Protein PhosphatasePPM1EServes to Maintain Insulin Secretion in Type 2 Diabetes
Sevda Gheibi, Luis Rodrigo Cataldo, Alexander Hamilton, Mi Huang, Sebastian Kalamajski, Malin Fex, Hindrik Mulder
Diabetes.2023; 72(4): 455. CrossRef - Metformin restores prohormone processing enzymes and normalizes aberrations in secretion of proinsulin and insulin in palmitate‐exposed human islets
Quan Wen, Azazul Islam Chowdhury, Banu Aydin, Mudhir Shekha, Rasmus Stenlid, Anders Forslund, Peter Bergsten
Diabetes, Obesity and Metabolism.2023; 25(12): 3757. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Metformin disrupts insulin secretion, causes proapoptotic and oxidative effects in rat pancreatic beta‐cells in vitro
Maíra M.R. Valle, Eloisa Aparecida Vilas‐Boas, Camila F. Lucena, Simone A. Teixeira, Marcelo N. Muscara, Angelo R. Carpinelli
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Protection by metformin against severe Covid-19: An in-depth mechanistic analysis
Nicolas Wiernsperger, Abdallah Al-Salameh, Bertrand Cariou, Jean-Daniel Lalau
Diabetes & Metabolism.2022; 48(4): 101359. CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Overexpression of miR-297b-5p Promotes Metformin-Mediated Protection Against Stearic Acid-Induced Senescence by Targeting Igf1r
Qingrui Zhao, Shenghan Su, Yuqing Lin, Xuebei Li, Lingfeng Dan, Yunjin Zhang, Chunxiao Yang, Xiaohan Li, Yimeng Dong, Chenchen Geng, Changhao Sun, Xia Chu, Huimin Lu
SSRN Electronic Journal .2022;[Epub] CrossRef - Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Domenico Conza, Paola Mirra, Gaetano Calì, Luigi Insabato, Francesca Fiory, Francesco Beguinot, Luca Ulianich
Cells.2021; 10(5): 1067. CrossRef - NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction
Suma Elumalai, Udayakumar Karunakaran, Jun-Sung Moon, Kyu-Chang Won
Cells.2021; 10(7): 1573. CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Different Effects of Metformin and A769662 on Sodium Iodate-Induced Cytotoxicity in Retinal Pigment Epithelial Cells: Distinct Actions on Mitochondrial Fission and Respiration
Chi-Ming Chan, Ponarulselvam Sekar, Duen-Yi Huang, Shu-Hao Hsu, Wan-Wan Lin
Antioxidants.2020; 9(11): 1057. CrossRef - Metformin Reduces Lipotoxicity-Induced Meta-Inflammation in β-Cells through the Activation of GPR40-PLC-IP3 Pathway
Ximei Shen, Beibei Fan, Xin Hu, Liufen Luo, Yuanli Yan, Liyong Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef
- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide

 KDA
KDA
 First
First Prev
Prev





